On May 9th, the research team of Yu Rao from the School of Pharmaceutical Sciences, Haitao Li from the School of Medicine, and Pilong Li from the School of Life Sciences at Tsinghua University jointly published their research results. In this study, the authors utilized the efficient, rapid, reversible, and dynamic degradation characteristics of targeted protein degradation technology (PROTAC) to explore the formation characteristics of related biomolecular condensates by degrading epigenetic regulatory factors (BRD4), monitored changes in the phase behavior and function of target proteins induced by PROTAC using immunofluorescence staining and high-throughput sequencing, and revealed the functional association between BRD4 and other components in the condensate during this process. This is the first application of PROTAC in the study of liquid-liquid phase separation (LLPS), providing new methods and insights for solving critical problems in the field.
Targeted protein degradation technology (PROteolysis TArgeting Chimeras, PROTAC) is a rapidly developing novel protein degradation strategy in recent years. The basic principle of PROTAC is to use bifunctional small molecules to induce ubiquitination of the target protein through the ubiquitin-proteasome system, thereby achieving degradation of the target protein. Since the first targeted androgen receptor PROTAC molecule ARV-110 entered clinical research in 2019, the field of PROTAC has entered a period of rapid development.
Liquid-liquid phase separation (LLPS) is the basis for biomolecular aggregation to form membraneless organelles in eukaryotic cells, as well as an important mechanism for cellular localization. Currently, establishing the relationship between phase separation phenomena and biological function is an important scientific issue in this field. Phase separation is highly dependent on systematic quantification, and the concentration at which each component forms LLPS is particularly crucial, requiring efficient, rapid, and dynamic perturbation techniques.
In addition, there is an urgent need to establish new methods to avoid false positives in vitro experiments, explore phase separation states under physiological conditions, analyze the transition of scaffold proteins and client protein functions in condensates. Existing technical means such as CRISPR-Cas9/RNA interference (RNAi) genetic tools face many challenges in directly studying phase separation in vivo. Gene editing methods have irreversible effects for DNA modification and it is difficult to study intermediate states of protein encoding. RNAi is not enough powerful tool because of its long effective time, which is inadequate to study dynamic phase separation processes in vivo. Although small molecule inhibitors can affect the formation of phase separation, they often act on active/enzyme sites, while phase separation is often induced by other protein functions such as scaffold domains, leading to insufficient research. At the same time, the functional effects of small molecule inhibitors often lead to upregulation feedback of target proteins. The field of phase separation still lacks efficient methods for directly interfering with LLPS in wild-type cell lines.
Through the combined use of PROTAC, immunofluorescence staining, quantitative calculations and other technologies, researchers have established a "PROTAC-target protein-LLPS" research method targeted at intracellular BRD4 condensates. The researchers used 100 nM of ZXH-3-26 to determine the endogenous BRD4 degradation kinetics in HeLa cells. They found that the PROTAC molecule targeting BRD4 (ZXH-3-26) could rapidly degrade BRD4 and its condensates at low concentrations and short times (Figure 1). After 30 minutes of BRD4-PROTACs treatment, a significant decrease in BRD4 protein levels was observed, and after 4 hours, BRD4 protein was completely degraded, while the amount of BRD4 condensates also decreased sharply. This indicates that PROTAC can serve as a tool for rapid perturbation of condensate components.
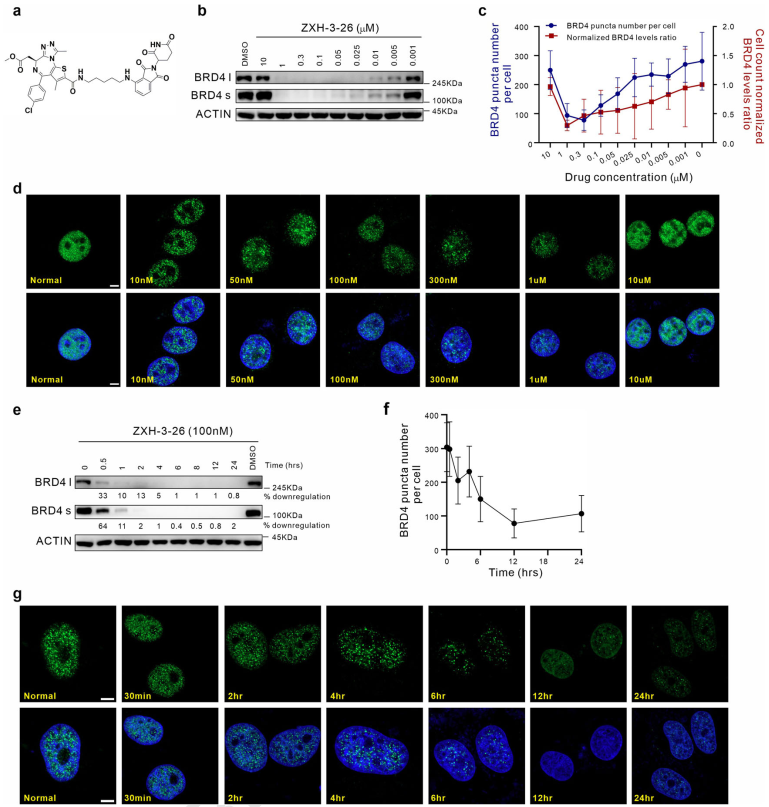
Figure 1. Establishment of the "PROTAC-target protein-LLPS" research method.
Subsequently, the researchers further explored the potential of using PROTAC for LLPS research by utilizing its reversibility. Interestingly, through wash-out experiments, they found that 24 hours after BRD4-PROTACs were removed, the number of endogenous BRD4 condensates had almost returned to the original levels, while at this time, the protein level of BRD4 had just begun to show signs of recovery (Figure 2). This suggests that the recovery of BRD4-related condensates may occur prior to the recovery of BRD4 protein levels, which is a phenomenon discovered for the first time in the application of PROTAC in LLPS research.
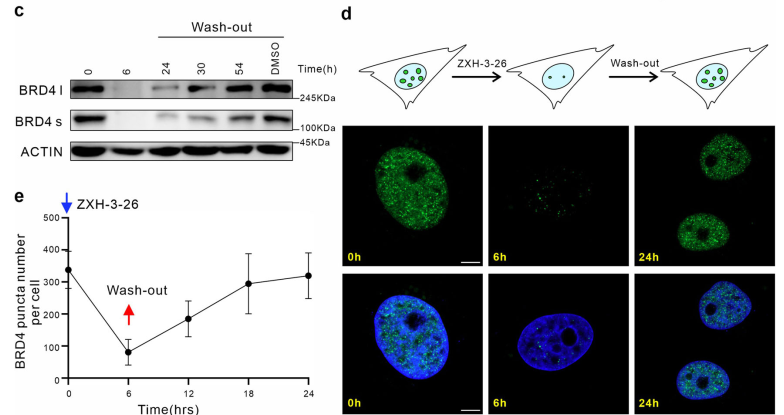
Figure 2. The reversibility of BRD4-PROTACs facilitates LLPS research
To explore the interesting phenomenon, the researchers further carried out multi-omics analysis to study its possible physiological and functional significance. Gene expression profiles of control groups, BRD4-PROTACs treated for 6 hours, wash-out for 18 hours, and wash-out for 42 hours were explored through RNA-seq and Cut&Tag-seq, as well as the enrichment changes of BRD4 in TSS regions, enhancer regions, and super-enhancer regions. The data showed that the gene expression profile of the wash-out 42-hour group had already recovered to a level similar to that of the control group. More surprisingly, as the degrader was removed, BRD4 quickly rebounded and preferred to occupy the super-enhancer regions, especially the super-enhancer regions of some key cancer genes and cell cycle genes (Figure 3). This result shows that PROTAC can be a powerful tool for studying the physiological and pathological functions of biomolecular condensates, and its rapid and reversible degradation is a key advantage as an LLPS research tool.

Figure 3. BRD4-PROTACs degradation and wash-out experiments
The multivalent interactions between components in biomolecular condensates are the basis of LLPS, and analyzing the physical and functional interactions among key protein components is a critical issue in the field of LLPS. Thus, based on BRD4-PROTACs, researchers explored the phase separation changes of other important components (MED1/CYCT1/p300) in BRD4-related condensates. The researchers found that under the action of BRD4-PROTACs, endogenous MED1 and CYCT1 condensates significantly decreased within 6 hours, but then recovered to nearly the same level as the control group after 12 hours, while p300, as an upstream molecule of BRD4, was not affected (Figure 4). In addition, they discovered for the first time that BRD3 played a compensatory role in the recovery of MED1 and CYCT1 condensates as BRD4 degraded. Targeting BRD4 with PROTAC not only provides a new effective tool for studying BRD4 phase separation but also offers a new strategy for analyzing the molecular mechanism and kinetics of BRD4 phase separation.
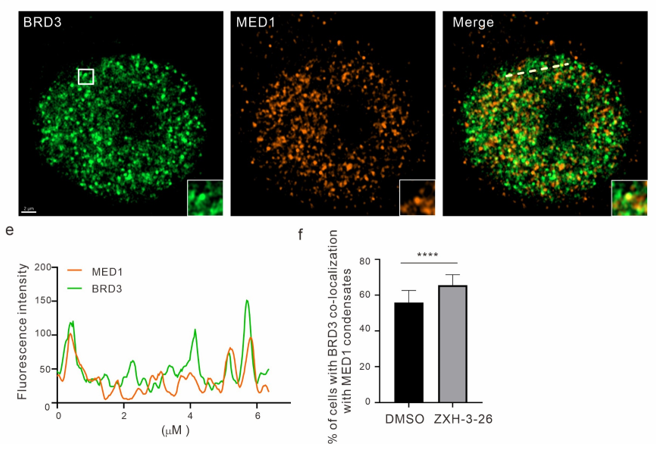
Figure 4. Exploration of the key functions of MED1/CYCT1/p300/BRD3 in BRD4 phase separation through BRD4-PROTACs
In summary, in this study, researchers first established the "PROTAC-target protein-LLPS" research method to explore the precise mechanisms of intracellular protein-protein interactions and protein spatiotemporal regulation related to LLPS from multiple dimensions. In LLPS research, the PROTAC-based research method is rapid, efficient, and reversible, and is superior to traditional genetic tools for disturbing target genes. Moreover, compared with small molecules commonly used for dissolving condensates (such as 1,6-hexanediol), PROTAC is more specific and targeted. In addition, combining this method with multi-omics techniques can not only explore the interaction and functional linker between components of condensates but also provide a possibility to establish relationships between phase separation and biological functions. It is worth mentioning that the PROTAC technology is considered a groundbreaking tool that aids in the development of small molecule drugs, and it is expected to play an important role in the treatment of diseases caused by abnormal phase separation.
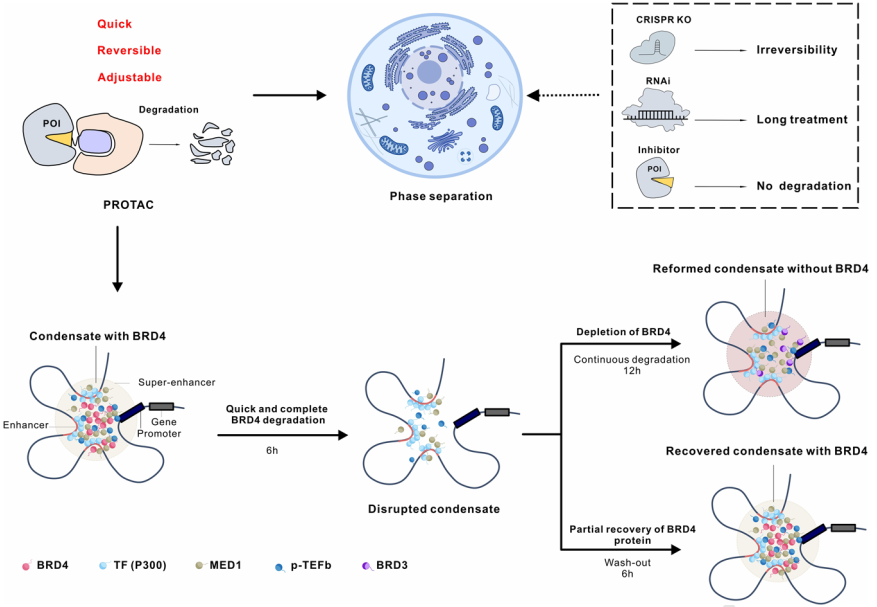
Figure 5. Working model of BRD4-PROTACs
Recently, the above-mentioned research paper entitled "BRD4-PROTACs as a unique tool to study biomolecular condensates" was published in the journal Cell Discovery.
Shi Yi (CLS program, 2020) from Prof. Yu Rao lab (School of Pharmaceutical Sciences) and Liao Yuan (PTN program,2020) from Prof. Haitao Li lab (School of Medicine), are co-first authors of the paper. Qianlong Liu, Zhihao Ni, Zhenzhen Zhang, participated in this work. Prof. Pilong Li, Prof. Haitao Li, and Prof. Yu Rao are corresponding authors of the paper. Prof. Wei Wu, Prof. Haiteng Deng, Prof. Jianyang Zeng and Dr. Minglei Shi provided valuable advice and guidance for this paper. And technical support was provided by the Cell Imaging Center and the Nikon Imaging Center at Tsinghua University. This research was supported by the National Natural Science Foundation of China and the National Key Research and Development Program of China.
Editor: Li Han

