Phycobilisomes (PBSs), the largest light-harvesting complexes (LHCs) known so far, are the major light-harvesting antennae in cyanobacteria and red algae. They are located on the matrix side of the thylakoid membrane and efficiently transfer the captured sunlight to the photosystems II and I (PSII and PSI ) to induce the conversion of light energy into chemical energy, a process called photosynthesis. While many photosynthetic protein complex structures have been determined through in vitro purification, their natural interactions and energy transfer pathways within cells remain unclear.
The research group led by Sen-Fang Sui from the School of Life Sciences at Tsinghua University has been dedicated to exploring the structure of phycobilisomes. In 2017, the team published an article in Nature, reporting the first structure of the complete phycobilisomes from the red alga Griffithsia pacifica at a resolution of 3.5 Å via the use of cryo-electron microscopy. This discovery revealed the complex and refined assembly mechanism of phycobilisomes. Furthermore, the team published another article in Nature in 2020, reporting the structure of phycobilisomes from the red alga Porphyridium purpureum with a higher resolution of 2.8 Å, which uncovered the energy transfer mechanism within phycobilisomes. This achievement is considered a significant breakthrough in the field of photosynthesis.
Recently, Sen-Fang Sui’s group and Hong-Wei Wang’s group of the School of Life Sciences, Tsinghua University, along with Xinzheng Zhang’s group from the Institute of Biophysics, Chinese Academy of Sciences, collaborated to report the first in situ structure of the red algal PBS–PSII–PSI–LHC megacomplex. This work is a milestone in the field of photosynthesis as it provides a solid structural foundation for understanding the assembly mechanism of PBS–PSII–PSI–LHC megacomplex in its native cellular state, as well as the efficient energy transfer mechanism from phycobilisome to PSII and PSI.
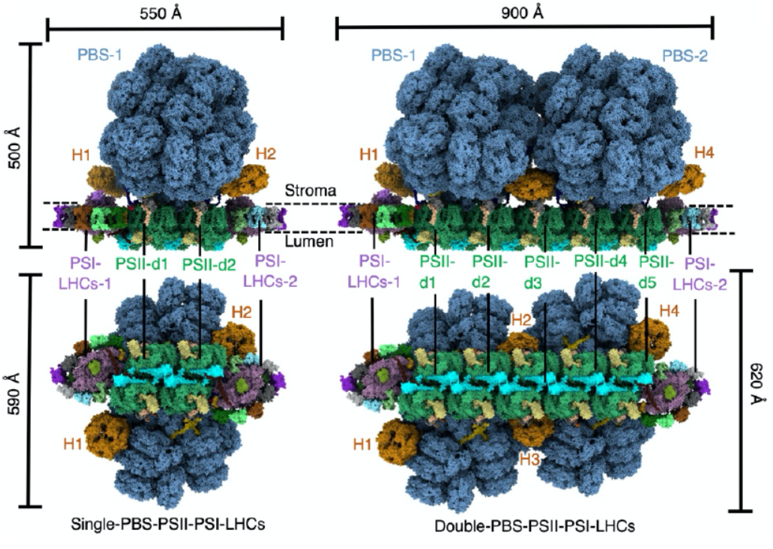
Figure 1. The overall structures of two types of assembly of PBS–PSII– PSI–LHC megacomplex
Based on previous researches on phycobilisomes, the research team selected Porphyridium purpureum as the target organism for the in situ high-resolution structural analysis. By combining cryo-focused ion beam (cryo-FIB), cryo-electron tomography (cryo-ET), subtomogram averaging, and in situ single-particle analysis (isSPA) methods, the team resolved the in situ structures of two types of assembly of the PBS–PSII–PSI–LHC megacomplex, single PBS–PSII–PSI–LHC and double PBS–PSII–PSI–LHC, at resolutions of 3.3 Å and 4.3 Å, respectively (Figure 1). Most notably, these structures reveal some novel protein molecules, which are essential to the assembly of the megacomplex, but have not been previously observed in purified and isolated samples. Structural analysis discovers that four linking proteins (LRC2, LRC3, LPP1, and LPP2), along with LCM and ApcD, are involved in the interaction between phycobilisome and PSII, enabling the red algal phycobilisome to stably bind to PSII in vivo.
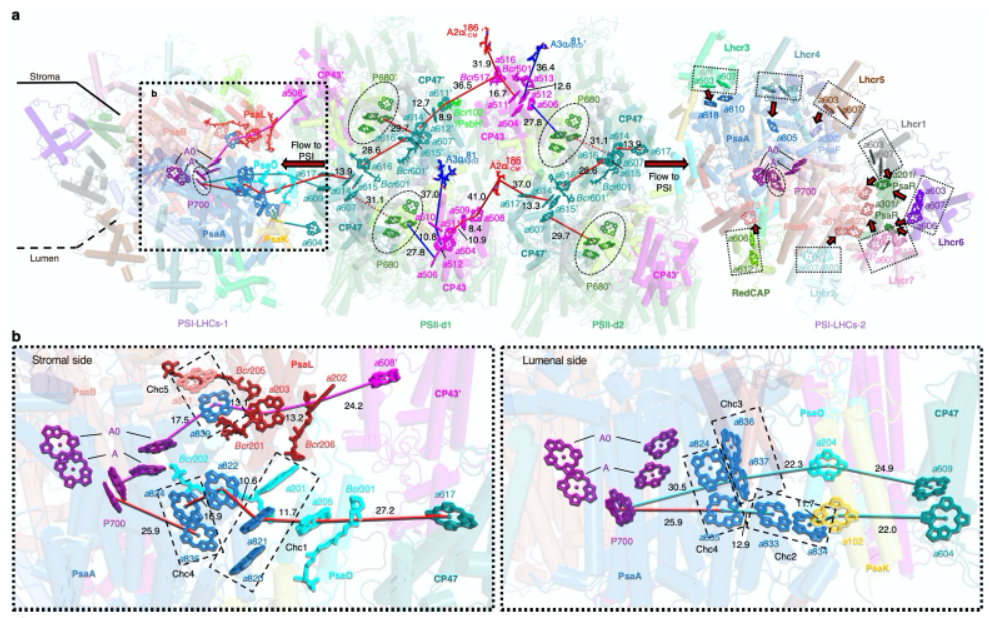
Figure 2. Key pigment arrangements and possible energy transfer pathways in in situ PBS–PSII– PSI–LHC megacomplex
Photosynthetic organisms have evolved sophisticated mechanisms to cope with the constantly fluctuating light conditions in nature. In cases where the excitation energy accumulated by PSII is excessive, the light-harvesting antenna transfers the energy to PSI to redistribute it and protect PSII from damage. Current understanding suggests that phycobilisome in cyanobacteria and red algae can transfer energy to PSI through direct phycobilisome → PSI pathway and indirect phycobilisome → PSII → PSI pathway. However, due to the lack of structural information, there has been ongoing controversy over the energy transfer mechanism between phycobilisome and PSI. The findings from the in situ structure of the PBS–PSII–PSI–LHC megacomplex provide a new perspective on this debate. The research has shown that red algal phycobilisome exclusively interacts with PSII, indicating that the light energy captured by phycobilisome is first transferred to PSII and then subsequently conveyed to PSI through a PSII-mediated process, consistent with the energy spillover model (Figure 2). Additionally, the team discovered that both PSII and PSI contain special arrangements of tightly packed chlorophyll clusters, which can form excitonic coupling states via π electrons in the conjugated rings, reducing the energy level to ensure efficient energy transfer (Figure 2). Taken together, these findings demonstrate that red algae transfer energy to PSI using the indirect phycobilisome → PSII → PSI mode, thereby redistributing the energy between PSII and PSI. At the same time, this study also provides new theoretical support for the development of artificial photosynthesis.
This work was published in Nature with the title of "In situ structure of the red algal phycobilisome–PSII–PSI–LHC megacomplex". Professors Sen-Fang Sui and Hong-Wei Wang of the School of Life Sciences, and Researcher Xinzheng Zhang of the Institute of Biophysics, Chinese Academy of Sciences are co-corresponding authors of this paper. Xin You, a doctoral student in the School of Life Sciences, Dr. Xing Zhang from the School of Life Sciences, Dr. Jing Cheng from the Institute of Biophysics, Chinese Academy of Sciences, and Dr. Yanan Xiao from Southern University of Science and Technology are co-first authors. Other authors include Dr. Jianfei Ma and Research-Track Associate Professor Shan Sun from the School of life Sciences. This research was supported by the National Natural Science Foundation of China, the National Basic Research Program, the National Key R&D Program of China, and the Xplorer Prize.
Link to the paper: https://www.nature.com/articles/s41586-023-05831-0
Editor: Li Han

